Why a new directive?
The previous version of the DWD, established in 1998, emphasized the importance of maintaining the quality of drinking water and preventing any adverse health effects. The European Commission cites the following reasons for the new DWD:
- Updating existing safety standards and developing an 'emerging substances' watchlist.
- Introduction of a 'risk-based approach' (RBA) covering the entire supply chain.
- Access to water: an obligation for member states to improve or maintain access to safe drinking water for everyone, especially vulnerable and marginalized groups.
- Provisions regarding substances and materials/products in contact with drinking water (Article 11 DWD).
- Greater transparency for consumers (public information).
Timelines
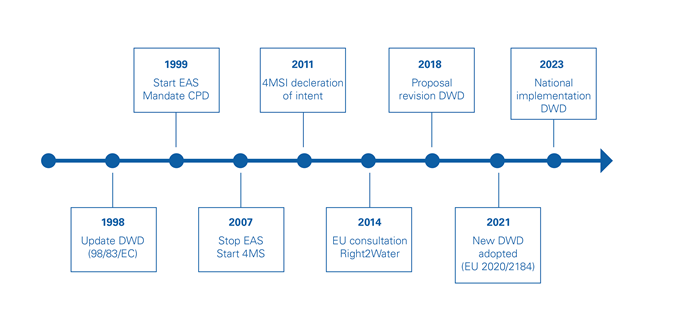
DWD - history
The new Drinking Water Directive did not come out of nowhere. It is the recast of the old 1998 DWD, taking the developments of the last decades into account. This includes the EAS and 4MSI results and the Right2Water initiative.
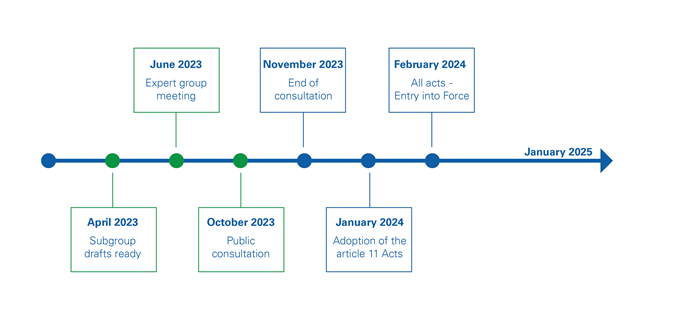
DWD - next steps
With the public consultation started, the next steps for the Implementing and Delegated Acts that make up the EU approval system for product inContact with drinking water are shown below. After closure of the consultation and resulting changes to the drafts (if any) the Acts will be published in the Official Journal of the European Union. 20 days later, the enter into force.
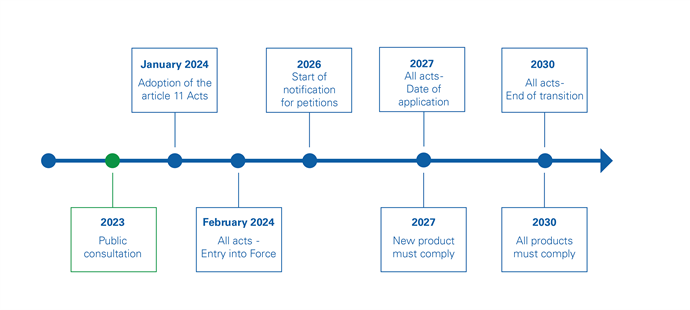
DWD - transition
With the entry into force of the Acts, the transition of the current situation of national approvals will transition into the EU system. Starting in 2027, products entering the EU market will need to comply. Products with existing national approvals have until 2030 (or possibly 2032) to comply.